- source: CentryMed
- author: CentryMed
-
2025.07.23
April 25, 2025 – CentryMed announced today that the U.S. Food and Drug Administration (FDA) has granted Investigational New Drug (IND) clearance to CMDE005, its recombinant human anti-EGFR-CD3 enzyme-controlled bispecific antibody. CMDE005, developed using CentryMed’s proprietary bispecific antibody platform proBiTE, is intended for the treatment of various EGFR-positive advanced solid tumors.
This milestone follows the drug candidate’s clinical trial approval from China’s National Medical Products Administration (NMPA) on September 12, 2024. As of the end of Q1 2025, patient enrollment is ongoing in the fourth dose cohort of the Phase I clinical trial, placing CMDE005’s clinical progress among the global leaders for this class of therapeutics.
01
About CMDE005 (Enzyme-Controlled TCE Technology)
CMDE005 is an anti-EGFR×CD3 bispecific antibody utilizing a "masking peptide" to block CD3 binding activity. In the peripheral blood and normal tissues, the anti-CD3 arm of CMDE005 remains inactive. However, upon reaching the tumor microenvironment, tumor-specific proteases (such as uPA) cleave specific sites on CMDE005. This cleavage releases the masking peptide, unblocking the CD3 binding function and converting CMDE005 into its active EGFR×CD3 bispecific antibody state. This mechanism enables targeted anti-tumor activity while minimizing off-tumor toxicity against normal tissues, resulting in high tumor specificity.
Recent reports indicate that innovative biotech companies Janux and Vir are developing similar "enzyme-controlled" candidates in early clinical development, showing preliminary promising disease control rates and safety in advanced solid tumors. Currently, no enzyme-controlled bispecific antibody product is approved globally. CentryMed’s CMDE005 is among the clinical frontrunners in this field, positioning the company to potentially lead in TCE therapy for solid tumors.
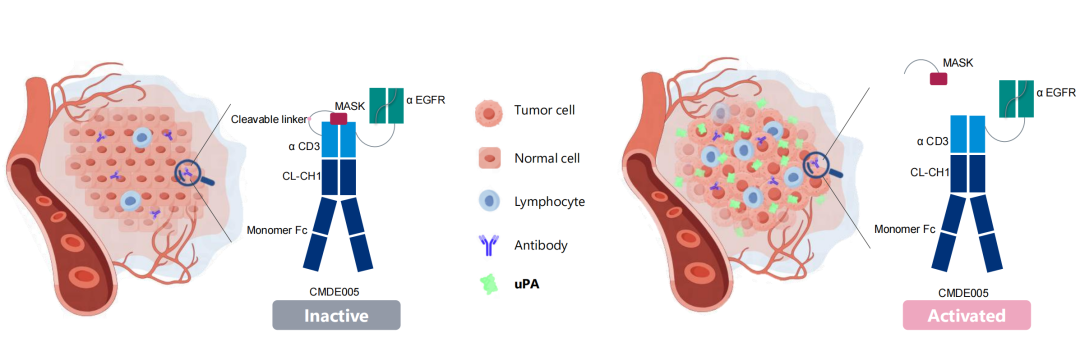
*(Figure 1: Schematic of CentryMed's First-in-Class Enzyme-Controlled Platform)*
02
About CentryMed’s Enzyme-Controlled Technology Platform (Pro-BiTE)
Since its founding in 2017, CentryMed has strategically focused on T-cell engager (TCE) antibodies. Driven by clinical needs, the company is committed to developing TCE-related therapies for cancer and autoimmune diseases. Through sustained technological innovation and the successful development of the EGFR×CD3 program, CentryMed has established its robust Pro-BiTE platform.
The Pro-BiTE platform incorporates masking peptides containing protease-cleavable linkers at the target-binding sites of the antibody. These peptides effectively block the antibody's activity in normal tissues and the peripheral circulation. When the antibody accumulates in the tumor microenvironment, tumor-specific proteases – such as urokinase-type plasminogen activator (uPA) and matrix metalloproteinases (MMPs) – recognize and cleave specific sites between the masking peptide and the antibody molecule. Cleavage releases the masking peptide, restoring the antibody’s target-binding activity and its anti-tumor function. This design significantly reduces off-tumor toxicity caused by non-specific killing of normal tissues, conferring high tumor-targeting specificity. This mechanism dramatically improves the safety profile and therapeutic window of TCE antibodies, offering a potential major breakthrough in solid tumor treatment and addressing significant unmet medical needs.
CMDE005 is the first product derived from this platform. It features convenient clinical administration (direct intravenous infusion) and broad-spectrum anti-tumor activity. As an immunotherapy, CMDE005 has the potential to improve the depth and duration of responses in responding patients compared to existing standard treatments, potentially enhancing overall survival benefit.
The FDA IND clearance for CMDE005 marks the achievement of dual IND approvals in both China and the U.S. This milestone validates CentryMed’s proprietary technology platform and R&D capabilities, strengthens the international recognition and data interoperability of the platform, and accelerates the initiation of global multi-center clinical trials. It significantly advances CentryMed’s international strategy and the global development path for its drug candidates. Through resource integration, risk diversification, and strategic capital deployment, this achievement substantially enhances the clinical and commercial value of the CMDE005 program and further solidifies CentryMed’s position as a global leader in bispecific antibody platforms.